.png)
FDAs Framework for Regulating Regenerative Medicine Will Improve Oversight | The Pew Charitable Trusts
.png)
FDAs Framework for Regulating Regenerative Medicine Will Improve Oversight | The Pew Charitable Trusts
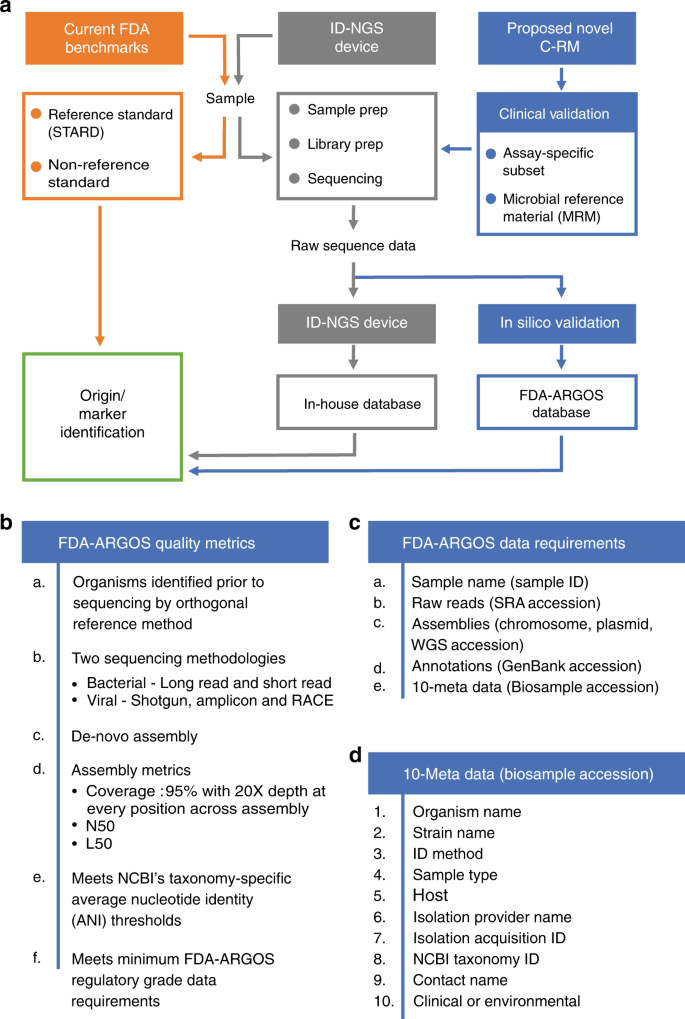
FDA-ARGOS is a database with public quality-controlled reference genomes for diagnostic use and regulatory science | Nature Communications








![Full text] Optimal management of atypical hemolytic uremic disease: challenges an | IJNRD Full text] Optimal management of atypical hemolytic uremic disease: challenges an | IJNRD](https://www.dovepress.com/cr_data/article_fulltext/s215000/215370/img/IJNRD_A_215370_O_F0003g.jpg)

![Full text] Optimal management of atypical hemolytic uremic disease: challenges an | IJNRD Full text] Optimal management of atypical hemolytic uremic disease: challenges an | IJNRD](https://www.dovepress.com/cr_data/article_fulltext/s215000/215370/img/IJNRD_A_215370_O_F0004g.jpg)